Dongsung Pharmaceutical Advances Ponogen for Cancer Treatment
Approval for Phase 2 Trials Marks Progress in Photodynamic Therapy Development
- Dongsung Pharmaceutical
- Ponogen
- photosensitizer
- clinical trials
- photodynamic therapy (PDT)
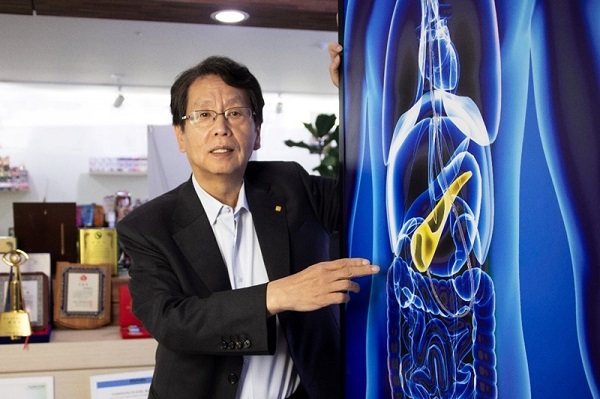
Phonogen is Dongsung Pharmaceutical's self-developed photosensitizer that selectively eliminates cancer cells with light-responsive properties, sparing normal cells.
Dongsung Pharmaceutical's Phase 2 trial assesses Ponogen (DSP1944) injection's safety and efficacy in photodynamic therapy (PDT) as adjunctive treatment for unresectable locally advanced pancreatic cancer patients undergoing chemotherapy.
Dongsung Pharmaceutical plans to accelerate Phonogen’s clinical trials and apply for photodynamic diagnosis (PDD) trials for peritoneal cancer, solidifying its leadership in photodynamic therapy (PDT) and diagnosis (PDD) in South Korea.
Dongsung Pharmaceutical's Ponogen (DSP1944) gains traction with consecutive listings in SCI-level journals, reflecting outstanding results. Additionally, the company engages in international discussions for license withdrawal, anticipating advantageous negotiation positions upon clinical approval.
